The mobile health space is growing: about half of the 3.4 billion smartphone and tablet users will have downloaded mobile health apps by 2018, according to some estimates. And there’s no shortage of apps: there were 165,000 health apps available in 2015, according to reports. That number has almost certainly grown since, but very few health apps have been approved for medical purposes by the FDA; FDA approval requires clinical evidence and that’s expensive. New drugs cost about $1 billion to develop, but apps qualify as devices, so approval for them is cheaper at just $31 million to $350 million.
The European inspection and certification company Tüv Süd gave Natural Cycles a CE certification in February, which means the app is now considered a medical device for contraception in Europe. To get the CE certification, Scherwitzl says the app has repeatedly demonstrated in a series of clinical studies that it improves the effectiveness of traditional planning methods. Notified bodies are companies like Tüv, which certify high-risk medical devices. EU member states pick the notified bodies, which are organizations that assess whether medical devices meet requirements set out in legislation. Scherwitzl says Natural Cycles followed the same approval path as the Durex Condom.
“At the core of any regulatory certification is strong, clinical evidence that the product works as intended,” Scherwitzl says. “Our ambition is to have Natural Cycles certified in every country of the world.” He and his wife, particle physicist Elina Berglund — part of the Nobel Prize-winning team that discovered the Higgs boson — founded the company together. Though she’d used a hormonal implant as birth control for years, they wanted to switch to the rhythm method to avoid pregnancy, just in case Berglund did want to get pregnant later.
As for plans for US approval, Scherwitzl says the process is similar to the one in Europe. “You submit your dossier, including all your clinical data, to the FDA and they review it and approve it if it meets their standards,” he says.
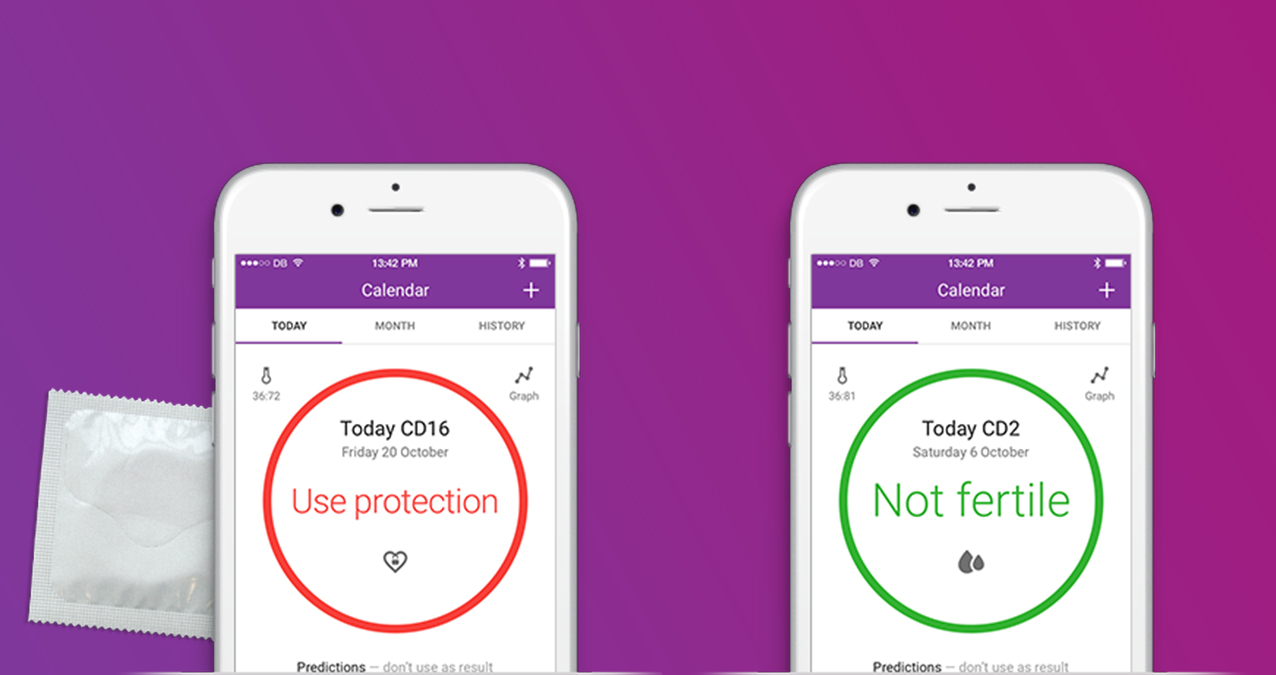
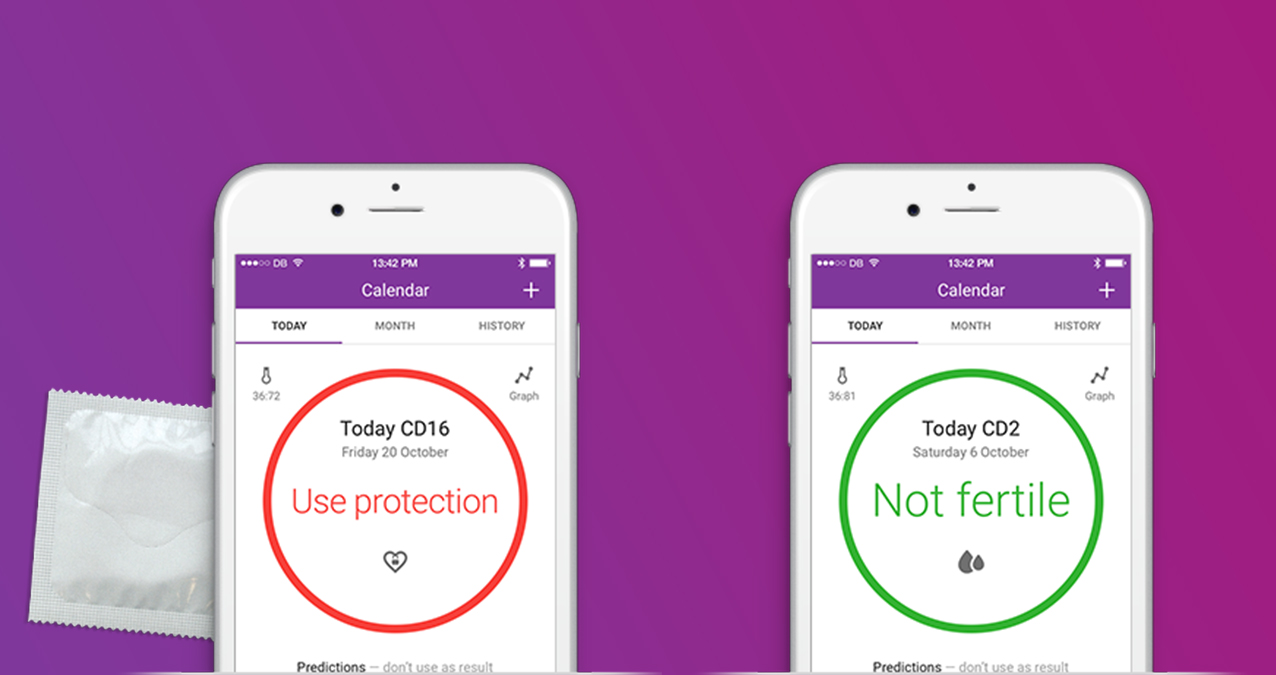
The app works like this: each morning, users measure their temperature, logging it into the app. Natural Cycles app uses an algorithm to calculate if the day is green, meaning not fertile, or red, when fertility is likely and using protection is recommended. The Swedish-based company charges $52 for a yearly plan, which includes a thermometer, or £5.99 ($7.80) per month. The company says its clinical studies have shown that the app is effective for contraception, “comparable to the contraceptive pill,” and it has over 300,000 users in 161 countries.

Howver, the app has it’s limitations and doesn’t prevent STDs. It doesn’t stop users from having unprotected sex during their fertile period, either. In fact, about half the women in a study of Natural Cycles got pregnant because they had unprotected sex while the app said they were fertile. A condom could have prevented that.
If apps like Natural Cycles are to be regulated, they would require evidence from rigorous study, says Victoria Jennings, director of the Institute for Reproductive Health at Georgetown University, in an email.
“The published research (to date) does not meet contraceptive efficacy standards,” Jennings says. “It may well be that such a study has been done, but it is not available in the published literature.”
The studies involved women who were using the app without “any interactions with health care professionals,” says Scherwitzl. He claims the entire pharmaceutical industry is headed toward this model, “because regulators demand it.”
Real-world data — which is collected from sources away from normal clinical trials — can “provide powerful insight into the benefits and risks of medical devices, including how they are used by health care providers and patients,” says Deborah Kotz, an FDA spokeswoman, in an email. The FDA is “working to implement” a national evaluation system for health technologies to make sure that data is high quality and reliable.
“If a fertility app meets the definition of a medical device and is intended to aid in becoming pregnant or preventing pregnancy, then it would be subject to FDA premarket review,” Kotz says.
She noted that the FDA has not yet cleared, granted, or approved any fertility apps for marketing.